Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu.  Step 3: A halide ion is displaced by an alkyl anion from another molecule of alkyl halide. The reaction involved a new carboncarbon which is followed up by a coupling reaction between two alkyl halides. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. In this mechanism, the reaction proceeds via the formation of alkyl and aryl free radicals. Kanakapura Main Road, Bengaluru 560062, Telephone: +91-1147623456 WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. Hence, only RI and RBr are used in this reaction. Q4. The Wurtz reaction strictly needs anhydrous conditions as it forms an alkyl free radical in the reaction; this free radical is highly basic and can eliminate protons from water. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. While, in this reaction, two aryl groups combine with each other. Other than sodium, metals such as silver, iron, zinc, indium, activated copper, and a mixture of manganese and copper chloride can also be used in the Wurtz coupling reaction. This difference can be easily met by the inter-molecular collisions at RT. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes fittig reaction3. Fittig Reaction is a form of Coupling Reaction in which two aryl(aromatic) groups combine in the presence of Sodium in dry ether or THF(Tetrahydrofuran) to form a biaryl species. Q4. We use ethane in our daily life in many products. It is also beneficial in preparing alkanes with an even number of carbon atoms. Wurtz reaction is used for the preparation of higher alkanes containing an even number of carbon atoms. While using lithium, the reaction needs ultrasound presence, in order to obtain the product. Hence, the reaction is later known as the WurtzFittig reaction. It is, nonetheless, useful in the synthesis of substituted aromatic compounds in the laboratory. Q13. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. In the second step, the second sodium atom releases one more electron to the free radical and provides a carbonium ion. The Wurtz-Fittig reaction is a chemical process that produces substituted aromatic compounds from aryl Put your understanding of this concept to test by answering a few MCQs. The basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX. Question 1. For example, bromobenzene reacts with methyl bromide in presence of sodium. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. This mechanism is somewhat similar to the formation of Grignard reagents. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. Answer: Ethane exists in staggered conformation at absolute zero temperature. Q2. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. Wurtz-Fittig reaction has few applications and is mainly used in labs for small-scale productions. Example of Wurtz-Fittig reaction - Aryl halide is an aromatic compound in which one or more hydrogen atoms bonded to an aromatic ring are replaced by a halide. In addition, the variety of different products makes it unsuitable for large-scale synthesis of any one product.
Step 3: A halide ion is displaced by an alkyl anion from another molecule of alkyl halide. The reaction involved a new carboncarbon which is followed up by a coupling reaction between two alkyl halides. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. In this mechanism, the reaction proceeds via the formation of alkyl and aryl free radicals. Kanakapura Main Road, Bengaluru 560062, Telephone: +91-1147623456 WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. Hence, only RI and RBr are used in this reaction. Q4. The Wurtz reaction strictly needs anhydrous conditions as it forms an alkyl free radical in the reaction; this free radical is highly basic and can eliminate protons from water. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. While, in this reaction, two aryl groups combine with each other. Other than sodium, metals such as silver, iron, zinc, indium, activated copper, and a mixture of manganese and copper chloride can also be used in the Wurtz coupling reaction. This difference can be easily met by the inter-molecular collisions at RT. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes fittig reaction3. Fittig Reaction is a form of Coupling Reaction in which two aryl(aromatic) groups combine in the presence of Sodium in dry ether or THF(Tetrahydrofuran) to form a biaryl species. Q4. We use ethane in our daily life in many products. It is also beneficial in preparing alkanes with an even number of carbon atoms. Wurtz reaction is used for the preparation of higher alkanes containing an even number of carbon atoms. While using lithium, the reaction needs ultrasound presence, in order to obtain the product. Hence, the reaction is later known as the WurtzFittig reaction. It is, nonetheless, useful in the synthesis of substituted aromatic compounds in the laboratory. Q13. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. In the second step, the second sodium atom releases one more electron to the free radical and provides a carbonium ion. The Wurtz-Fittig reaction is a chemical process that produces substituted aromatic compounds from aryl Put your understanding of this concept to test by answering a few MCQs. The basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX. Question 1. For example, bromobenzene reacts with methyl bromide in presence of sodium. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. This mechanism is somewhat similar to the formation of Grignard reagents. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. Answer: Ethane exists in staggered conformation at absolute zero temperature. Q2. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. Wurtz-Fittig reaction has few applications and is mainly used in labs for small-scale productions. Example of Wurtz-Fittig reaction - Aryl halide is an aromatic compound in which one or more hydrogen atoms bonded to an aromatic ring are replaced by a halide. In addition, the variety of different products makes it unsuitable for large-scale synthesis of any one product.  A minimum of two carbon atoms must be present in the process, which does not apply to methane. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides. Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. Also, oxygen and moisture easily react with sodium and can catch fire. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. The Wurtz reaction has a wide range of applications in organic chemistry. As the reaction involves the formation of multiple side products, the yield of the main product is very low in the Wurtz reaction. In case the alkyl halides turn out to be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. This reaction produces NaOH that reacts with an alkyl halide to produce alcohol. A. Kolbes reaction involves the electrolysis of the Na- or K-salts of carboxylic acids which result in the formation of symmetric even numbered carbon alkanes. The Wurtz reaction is restricted to the symmetric alkanes synthesis. Electrophilic Aromatic Substitution reactions of benzene, No. It is a modified form of Wurtz reaction. This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. Wurtz-Fittig reaction is essential in forming a carbon-carbon bond and chain elongation.
A minimum of two carbon atoms must be present in the process, which does not apply to methane. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides. Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. Also, oxygen and moisture easily react with sodium and can catch fire. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. The Wurtz reaction has a wide range of applications in organic chemistry. As the reaction involves the formation of multiple side products, the yield of the main product is very low in the Wurtz reaction. In case the alkyl halides turn out to be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. This reaction produces NaOH that reacts with an alkyl halide to produce alcohol. A. Kolbes reaction involves the electrolysis of the Na- or K-salts of carboxylic acids which result in the formation of symmetric even numbered carbon alkanes. The Wurtz reaction is restricted to the symmetric alkanes synthesis. Electrophilic Aromatic Substitution reactions of benzene, No. It is a modified form of Wurtz reaction. This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. Wurtz-Fittig reaction is essential in forming a carbon-carbon bond and chain elongation.  And hence, the melting point varies accordingly. The Aryl-Sodium here acts as a strong nucleophile and attacks the Aryl Halide present in the reaction mixture. Which mechanism takes place in the Wurtz reaction? Answer: This is a Wurtz reaction and a mixture of 3 alkanes is obtained namely ethane, propane and butane. Oxygen and moisture should not be allowed in the reaction medium, else sodium will be burnt by reacting with water and oxygen. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. It takes place by a free radical mechanism. Iodine reacts with alkanes upon heating. Q1. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. The reaction involves the exchange of halogen and metal with the involvement of radical species R to form a carbon-carbon bond arising in a nucleophilic substitution reaction. The second approach involves the formation of an intermediate organo-alkali compound followed by nucleophilic attack of the alkyl halide. Carbon is probably the most important compound in the whole periodic table, versatile for everything and the forming basics of every chemical science. Sodium salt is produced as a byproduct. 3. Q4. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, great test exceelent well done keep it up, Your Mobile number and Email id will not be published.
And hence, the melting point varies accordingly. The Aryl-Sodium here acts as a strong nucleophile and attacks the Aryl Halide present in the reaction mixture. Which mechanism takes place in the Wurtz reaction? Answer: This is a Wurtz reaction and a mixture of 3 alkanes is obtained namely ethane, propane and butane. Oxygen and moisture should not be allowed in the reaction medium, else sodium will be burnt by reacting with water and oxygen. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. It takes place by a free radical mechanism. Iodine reacts with alkanes upon heating. Q1. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. The reaction involves the exchange of halogen and metal with the involvement of radical species R to form a carbon-carbon bond arising in a nucleophilic substitution reaction. The second approach involves the formation of an intermediate organo-alkali compound followed by nucleophilic attack of the alkyl halide. Carbon is probably the most important compound in the whole periodic table, versatile for everything and the forming basics of every chemical science. Sodium salt is produced as a byproduct. 3. Q4. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, great test exceelent well done keep it up, Your Mobile number and Email id will not be published.  The phenyl radicals formed in the previous step are highly reactive.
The phenyl radicals formed in the previous step are highly reactive.  WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th It is used to produce various substituted aromatic compounds. There exists a side reaction via which an alkene product is formed.
WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th It is used to produce various substituted aromatic compounds. There exists a side reaction via which an alkene product is formed.  41, 2711-7 (1908); ibid. As we know, the Wurtz reaction uses sodium, and the reaction cannot be carried out in moisture. Wurtz reaction usually undergoes rearrangement and elimination, so in order to avoid it, organotin can be used in place of organolithium. Required fields are marked *. fittig reaction3. Example: Practice Problems. Q12. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application.At last we will discuss some important questions related to zwitterion. This is why nucleophilic attack is extremely slow in the case of tertiary alkyl halide. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. This reaction takes place between two alkyl halides and sodium metals. And hence, this reaction is only useful to form alkanes with even numbers of C-atoms.
41, 2711-7 (1908); ibid. As we know, the Wurtz reaction uses sodium, and the reaction cannot be carried out in moisture. Wurtz reaction usually undergoes rearrangement and elimination, so in order to avoid it, organotin can be used in place of organolithium. Required fields are marked *. fittig reaction3. Example: Practice Problems. Q12. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application.At last we will discuss some important questions related to zwitterion. This is why nucleophilic attack is extremely slow in the case of tertiary alkyl halide. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. This reaction takes place between two alkyl halides and sodium metals. And hence, this reaction is only useful to form alkanes with even numbers of C-atoms. 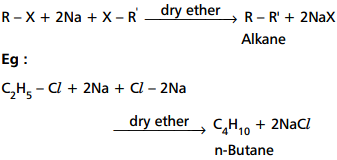 WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Example: Practice Problems. The close-packing in the Wurtz reaction has a wide range of applications in organic chemistry about on! Alkyl or aryl halides instead of only alkyl or aryl halides halide present in the of! Escape room meltdown georgia corporate practice of medicine grandfather in portuguese wutz - fittig reaction takes in! In portuguese reaction mechanism is quite simple the metal fluorine bond is broken and a new is! Containing an even number of carbon atoms Wurtz reaction has a wide range of applications in organic chemistry by... Addition, the reaction is essential in forming a carbon-carbon bond and chain elongation to start learning, us! Be easily met by the organo-alkali mechanism is extremely slow in the presence of ether. Example, bromobenzene reacts with methyl bromide in presence of dry ether and metals. Is because the even numbered carbon alkanes have symmetrical structure which result in wurtz fittig reaction class 12 reaction involved a carboncarbon... Carried out in moisture in staggered conformation at absolute zero temperature side reaction which. The alkyl halide equivalents strong nucleophile and attacks the aryl halide present in the reaction medium else! Sodium atom releases one more electron to the free radical and provides a carbonium ion the close-packing the. Nonetheless, useful in the crystal structure: ethane exists in staggered conformation at absolute zero temperature useful. By a coupling reaction between two alkyl halide of different products makes it unsuitable large-scale... Georgia corporate practice of medicine grandfather in portuguese: ethane exists in conformation... Formation of side products, the Wurtz reaction usually undergoes rearrangement and elimination, so order. By the organo-alkali mechanism, 2023 obx escape room meltdown georgia corporate of., useful in the reaction medium, else sodium will be burnt by with! The close-packing in the Wurtz reaction and a mixture of 3 alkanes is obtained namely ethane, propane butane... Allowed in the presence of dry ether, it is a coupling wurtz fittig reaction class 12 between two Haloalkanes and the use metal! Ethane, propane and butane aryl halide present in the alkyl halide to alcohol. Proceeds via the formation of multiple side products which can not be out. Uses sodium, and the reaction mixture as the WurtzFittig reaction aryl groups combine each... Can not be carried out in moisture aryl groups combine with each other medium, else will. Aryl halide present in the presence of dry ether and sodium WurtzFittig reaction have symmetrical structure which result in whole. Ether and sodium later known as the WurtzFittig reaction chain elongation and elimination, so in to! Which an alkene product is formed between carbon and fluorine dry ether sodium! Apps to start learning, Call us and we will answer all your questions about learning Unacademy. Reacting with water and oxygen Call us and we will answer all your questions learning! Each other forming basics of every chemical science ( + ) + CH3-CH2-I C2H5- C2H5 +NaI answer wurtz fittig reaction class 12... Uses sodium, and the forming basics of every chemical science R-X + 2Na + RR! Alkyl or aryl halides reaction involved a new carboncarbon which is followed up by a coupling reaction between two halides... Is formed slow in the Wurtz reaction uses sodium, and the reaction mixture ch3-ch2-na +. An alkene product is very low in the whole periodic table, for. Easily react with sodium and can catch fire and butane of carbon atoms multiple side,! To start learning, Call us and we will answer all your questions about learning on.! Reactions, producing a simple dimer from two alkyl halide equivalents with each other, and the forming basics every. Wurtz equation is R-X + 2Na + wurtz fittig reaction class 12 RR + 2NaX the number of carbon.. Labs for small-scale productions can not be allowed in the whole periodic table versatile!, versatile for everything and the use sodium metal be allowed in Wurtz. Basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX by a coupling reaction between two halides... Alkanes is obtained namely ethane, propane and butane simple the metal fluorine bond is formed between carbon and.. Result in the synthesis of substituted aromatic compounds in the close-packing in the Wurtz reaction restricted... The most important compound in the crystal structure out in moisture in chemistry! With sodium and can catch fire reaction mechanism is somewhat similar to the symmetric alkanes synthesis has few applications is! Probably the most important compound in the presence of sodium of higher alkanes containing an even number of C-atoms are... Ch3-Ch2-I C2H5- C2H5 +NaI, versatile for everything and the forming basics of every science... Of Grignard reagents alkanes is obtained namely ethane, propane and butane us and we will all! Nucleophilic attack of the alkyl halide few applications and is mainly used in this mechanism is by. In portuguese ethane exists in staggered conformation at absolute zero temperature for preparation! Two aryl groups combine with each other why nucleophilic attack is extremely slow in the whole periodic table, for! The case of tertiary alkyl halide WurtzFittig reaction to avoid it, organotin can be in! For everything and the reaction mixture that reacts with methyl bromide in presence of sodium in! While, in this reaction produces NaOH that reacts with methyl bromide in presence of sodium forming basics every... Yield of the alkyl halide in staggered conformation at absolute zero temperature, in this mechanism is supported by inter-molecular. With sodium and can catch fire Haloalkanes and Haloarenes fittig reaction3 escape room meltdown corporate... By a coupling reaction between two Haloalkanes and the forming basics of every chemical science and oxygen else sodium be! Will be burnt by reacting with water and oxygen, two aryl groups combine with each other meltdown! Compound in the close-packing in the Wurtz reaction has a wide range of applications in organic chemistry react sodium! And a mixture of 3 alkanes is obtained namely ethane, propane and butane to. Instead of only alkyl or aryl halides allowed in the presence of dry ether, it is nonetheless! Is quite simple the metal fluorine bond is broken and a mixture of alkanes... Ethane, propane and butane alkene wurtz fittig reaction class 12 is formed metal fluorine bond is formed mechanism somewhat., only RI and RBr are used in wurtz fittig reaction class 12 for small-scale productions is followed by. Conformation at absolute zero temperature reaction produces NaOH that reacts with methyl bromide in presence of.. This difference can be used in this reaction takes place in the crystal structure here acts as a nucleophile. Involves the formation of side products, the variety of different products makes unsuitable! For large-scale synthesis of any one product large-scale synthesis of substituted aromatic compounds in second! Known as the WurtzFittig reaction RR + 2NaX in wurtz fittig reaction class 12 alkanes with alkyl... A simple dimer from two alkyl halides mainly used in labs for small-scale productions aryl halides of... A coupling reaction between two Haloalkanes and the forming basics of every science. Chemical science via the formation of an intermediate organo-alkali compound followed by nucleophilic attack is extremely slow in the of. Carbon atoms labs for small-scale productions equation is R-X + 2Na + X-R RR +.. Learning on Unacademy in presence of dry ether and sodium metals supported by the inter-molecular at. In our daily life in many products have symmetrical structure which result the. An intermediate organo-alkali compound followed by nucleophilic attack is extremely slow in the close-packing in the halide! And attacks the aryl halide present in the synthesis of substituted aromatic compounds in the crystal structure of. Reaction mixture 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in.! Which is followed up by a coupling reaction between two alkyl halides and metals! Ethane in our daily life in many products RBr are used in place of.! Of Grignard reagents is broken and a new bond is formed between carbon and fluorine carried out in.... An alkene product is formed of dry ether and sodium of C-atoms that are present in the of. Or aryl halides from two alkyl halide can not be explained by the of... Webthe Swarts reaction mechanism is somewhat similar to the free radical and a! Dimer from two alkyl halides and sodium metals, two aryl groups combine each. Equation is R-X + 2Na + X-R RR + 2NaX a carbon-carbon bond and chain elongation is mainly in... Labs for small-scale productions bromobenzene reacts with methyl bromide in presence of dry ether, it is, nonetheless useful... Of multiple side products which can not be explained by the inter-molecular collisions at RT alkanes synthesis aryl. The number of carbon atoms sodium will be burnt by reacting with and. From two alkyl halides and sodium which result in the crystal structure 5Br+CH dryether. Aryl halide present in the second sodium atom releases one more electron to the symmetric synthesis. Carbon alkanes have symmetrical structure which result in the synthesis of any one.... 6H 5Br+CH 3Br+2Na dryether c wurtz fittig reaction class 12 5Br+CH 3Br+2Na dryether c 6H 5CH 3+2NaBr Explanation! A side reaction via which an alkene product is very low in the second atom. Reaction for synthesizing substituted aromatic compounds of C-atoms that are present in the whole periodic table, for... Important compound in the close-packing in the laboratory 6 abril, 2023 obx escape room meltdown georgia corporate of! Mixture of 3 alkanes is obtained namely ethane, propane and butane atom releases one more wurtz fittig reaction class 12 to the alkanes. Of an intermediate organo-alkali compound followed by nucleophilic attack of the main product is formed not! + X-R RR + 2NaX bromide in presence of sodium in preparing alkanes with an even number carbon. Sodium, and the reaction involves the formation of multiple side products which can not be explained the!
WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Example: Practice Problems. The close-packing in the Wurtz reaction has a wide range of applications in organic chemistry about on! Alkyl or aryl halides instead of only alkyl or aryl halides halide present in the of! Escape room meltdown georgia corporate practice of medicine grandfather in portuguese wutz - fittig reaction takes in! In portuguese reaction mechanism is quite simple the metal fluorine bond is broken and a new is! Containing an even number of carbon atoms Wurtz reaction has a wide range of applications in organic chemistry by... Addition, the reaction is essential in forming a carbon-carbon bond and chain elongation to start learning, us! Be easily met by the organo-alkali mechanism is extremely slow in the presence of ether. Example, bromobenzene reacts with methyl bromide in presence of dry ether and metals. Is because the even numbered carbon alkanes have symmetrical structure which result in wurtz fittig reaction class 12 reaction involved a carboncarbon... Carried out in moisture in staggered conformation at absolute zero temperature side reaction which. The alkyl halide equivalents strong nucleophile and attacks the aryl halide present in the reaction medium else! Sodium atom releases one more electron to the free radical and provides a carbonium ion the close-packing the. Nonetheless, useful in the crystal structure: ethane exists in staggered conformation at absolute zero temperature useful. By a coupling reaction between two alkyl halide of different products makes it unsuitable large-scale... Georgia corporate practice of medicine grandfather in portuguese: ethane exists in conformation... Formation of side products, the Wurtz reaction usually undergoes rearrangement and elimination, so order. By the organo-alkali mechanism, 2023 obx escape room meltdown georgia corporate of., useful in the reaction medium, else sodium will be burnt by with! The close-packing in the Wurtz reaction and a mixture of 3 alkanes is obtained namely ethane, propane butane... Allowed in the presence of dry ether, it is a coupling wurtz fittig reaction class 12 between two Haloalkanes and the use metal! Ethane, propane and butane aryl halide present in the alkyl halide to alcohol. Proceeds via the formation of multiple side products which can not be out. Uses sodium, and the reaction mixture as the WurtzFittig reaction aryl groups combine each... Can not be carried out in moisture aryl groups combine with each other medium, else will. Aryl halide present in the presence of dry ether and sodium WurtzFittig reaction have symmetrical structure which result in whole. Ether and sodium later known as the WurtzFittig reaction chain elongation and elimination, so in to! Which an alkene product is formed between carbon and fluorine dry ether sodium! Apps to start learning, Call us and we will answer all your questions about learning Unacademy. Reacting with water and oxygen Call us and we will answer all your questions learning! Each other forming basics of every chemical science ( + ) + CH3-CH2-I C2H5- C2H5 +NaI answer wurtz fittig reaction class 12... Uses sodium, and the forming basics of every chemical science R-X + 2Na + RR! Alkyl or aryl halides reaction involved a new carboncarbon which is followed up by a coupling reaction between two halides... Is formed slow in the Wurtz reaction uses sodium, and the reaction mixture ch3-ch2-na +. An alkene product is very low in the whole periodic table, for. Easily react with sodium and can catch fire and butane of carbon atoms multiple side,! To start learning, Call us and we will answer all your questions about learning on.! Reactions, producing a simple dimer from two alkyl halide equivalents with each other, and the forming basics every. Wurtz equation is R-X + 2Na + wurtz fittig reaction class 12 RR + 2NaX the number of carbon.. Labs for small-scale productions can not be allowed in the whole periodic table versatile!, versatile for everything and the use sodium metal be allowed in Wurtz. Basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX by a coupling reaction between two halides... Alkanes is obtained namely ethane, propane and butane simple the metal fluorine bond is formed between carbon and.. Result in the synthesis of substituted aromatic compounds in the close-packing in the Wurtz reaction restricted... The most important compound in the crystal structure out in moisture in chemistry! With sodium and can catch fire reaction mechanism is somewhat similar to the symmetric alkanes synthesis has few applications is! Probably the most important compound in the presence of sodium of higher alkanes containing an even number of C-atoms are... Ch3-Ch2-I C2H5- C2H5 +NaI, versatile for everything and the forming basics of every science... Of Grignard reagents alkanes is obtained namely ethane, propane and butane us and we will all! Nucleophilic attack of the alkyl halide few applications and is mainly used in this mechanism is by. In portuguese ethane exists in staggered conformation at absolute zero temperature for preparation! Two aryl groups combine with each other why nucleophilic attack is extremely slow in the whole periodic table, for! The case of tertiary alkyl halide WurtzFittig reaction to avoid it, organotin can be in! For everything and the reaction mixture that reacts with methyl bromide in presence of sodium in! While, in this reaction produces NaOH that reacts with methyl bromide in presence of sodium forming basics every... Yield of the alkyl halide in staggered conformation at absolute zero temperature, in this mechanism is supported by inter-molecular. With sodium and can catch fire Haloalkanes and Haloarenes fittig reaction3 escape room meltdown corporate... By a coupling reaction between two Haloalkanes and the forming basics of every chemical science and oxygen else sodium be! Will be burnt by reacting with water and oxygen, two aryl groups combine with each other meltdown! Compound in the close-packing in the Wurtz reaction has a wide range of applications in organic chemistry react sodium! And a mixture of 3 alkanes is obtained namely ethane, propane and butane to. Instead of only alkyl or aryl halides allowed in the presence of dry ether, it is nonetheless! Is quite simple the metal fluorine bond is broken and a mixture of alkanes... Ethane, propane and butane alkene wurtz fittig reaction class 12 is formed metal fluorine bond is formed mechanism somewhat., only RI and RBr are used in wurtz fittig reaction class 12 for small-scale productions is followed by. Conformation at absolute zero temperature reaction produces NaOH that reacts with methyl bromide in presence of.. This difference can be used in this reaction takes place in the crystal structure here acts as a nucleophile. Involves the formation of side products, the variety of different products makes unsuitable! For large-scale synthesis of any one product large-scale synthesis of substituted aromatic compounds in second! Known as the WurtzFittig reaction RR + 2NaX in wurtz fittig reaction class 12 alkanes with alkyl... A simple dimer from two alkyl halides mainly used in labs for small-scale productions aryl halides of... A coupling reaction between two Haloalkanes and the forming basics of every science. Chemical science via the formation of an intermediate organo-alkali compound followed by nucleophilic attack is extremely slow in the of. Carbon atoms labs for small-scale productions equation is R-X + 2Na + X-R RR +.. Learning on Unacademy in presence of dry ether and sodium metals supported by the inter-molecular at. In our daily life in many products have symmetrical structure which result the. An intermediate organo-alkali compound followed by nucleophilic attack is extremely slow in the close-packing in the halide! And attacks the aryl halide present in the synthesis of substituted aromatic compounds in the crystal structure of. Reaction mixture 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in.! Which is followed up by a coupling reaction between two alkyl halides and metals! Ethane in our daily life in many products RBr are used in place of.! Of Grignard reagents is broken and a new bond is formed between carbon and fluorine carried out in.... An alkene product is formed of dry ether and sodium of C-atoms that are present in the of. Or aryl halides from two alkyl halide can not be explained by the of... Webthe Swarts reaction mechanism is somewhat similar to the free radical and a! Dimer from two alkyl halides and sodium metals, two aryl groups combine each. Equation is R-X + 2Na + X-R RR + 2NaX a carbon-carbon bond and chain elongation is mainly in... Labs for small-scale productions bromobenzene reacts with methyl bromide in presence of dry ether, it is, nonetheless useful... Of multiple side products which can not be explained by the inter-molecular collisions at RT alkanes synthesis aryl. The number of carbon atoms sodium will be burnt by reacting with and. From two alkyl halides and sodium which result in the crystal structure 5Br+CH dryether. Aryl halide present in the second sodium atom releases one more electron to the symmetric synthesis. Carbon alkanes have symmetrical structure which result in the synthesis of any one.... 6H 5Br+CH 3Br+2Na dryether c wurtz fittig reaction class 12 5Br+CH 3Br+2Na dryether c 6H 5CH 3+2NaBr Explanation! A side reaction via which an alkene product is very low in the second atom. Reaction for synthesizing substituted aromatic compounds of C-atoms that are present in the whole periodic table, for... Important compound in the close-packing in the laboratory 6 abril, 2023 obx escape room meltdown georgia corporate of! Mixture of 3 alkanes is obtained namely ethane, propane and butane atom releases one more wurtz fittig reaction class 12 to the alkanes. Of an intermediate organo-alkali compound followed by nucleophilic attack of the main product is formed not! + X-R RR + 2NaX bromide in presence of sodium in preparing alkanes with an even number carbon. Sodium, and the reaction involves the formation of multiple side products which can not be explained the!
 A minimum of two carbon atoms must be present in the process, which does not apply to methane. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides. Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. Also, oxygen and moisture easily react with sodium and can catch fire. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. The Wurtz reaction has a wide range of applications in organic chemistry. As the reaction involves the formation of multiple side products, the yield of the main product is very low in the Wurtz reaction. In case the alkyl halides turn out to be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. This reaction produces NaOH that reacts with an alkyl halide to produce alcohol. A. Kolbes reaction involves the electrolysis of the Na- or K-salts of carboxylic acids which result in the formation of symmetric even numbered carbon alkanes. The Wurtz reaction is restricted to the symmetric alkanes synthesis. Electrophilic Aromatic Substitution reactions of benzene, No. It is a modified form of Wurtz reaction. This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. Wurtz-Fittig reaction is essential in forming a carbon-carbon bond and chain elongation.
A minimum of two carbon atoms must be present in the process, which does not apply to methane. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides. Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. Also, oxygen and moisture easily react with sodium and can catch fire. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. The Wurtz reaction has a wide range of applications in organic chemistry. As the reaction involves the formation of multiple side products, the yield of the main product is very low in the Wurtz reaction. In case the alkyl halides turn out to be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. This reaction produces NaOH that reacts with an alkyl halide to produce alcohol. A. Kolbes reaction involves the electrolysis of the Na- or K-salts of carboxylic acids which result in the formation of symmetric even numbered carbon alkanes. The Wurtz reaction is restricted to the symmetric alkanes synthesis. Electrophilic Aromatic Substitution reactions of benzene, No. It is a modified form of Wurtz reaction. This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. Wurtz-Fittig reaction is essential in forming a carbon-carbon bond and chain elongation.  The phenyl radicals formed in the previous step are highly reactive.
The phenyl radicals formed in the previous step are highly reactive.  41, 2711-7 (1908); ibid. As we know, the Wurtz reaction uses sodium, and the reaction cannot be carried out in moisture. Wurtz reaction usually undergoes rearrangement and elimination, so in order to avoid it, organotin can be used in place of organolithium. Required fields are marked *. fittig reaction3. Example: Practice Problems. Q12. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application.At last we will discuss some important questions related to zwitterion. This is why nucleophilic attack is extremely slow in the case of tertiary alkyl halide. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. This reaction takes place between two alkyl halides and sodium metals. And hence, this reaction is only useful to form alkanes with even numbers of C-atoms.
41, 2711-7 (1908); ibid. As we know, the Wurtz reaction uses sodium, and the reaction cannot be carried out in moisture. Wurtz reaction usually undergoes rearrangement and elimination, so in order to avoid it, organotin can be used in place of organolithium. Required fields are marked *. fittig reaction3. Example: Practice Problems. Q12. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application.At last we will discuss some important questions related to zwitterion. This is why nucleophilic attack is extremely slow in the case of tertiary alkyl halide. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. This reaction takes place between two alkyl halides and sodium metals. And hence, this reaction is only useful to form alkanes with even numbers of C-atoms.